Students probably are looking for assignment help for CHEM101: Diagnose The Acidity/ Alkalinity of Chemical Substances - “Acid Test” Chemistry Assessment. These types of assignment have never been easy for students to understand. But Sample Assignment is available now to with CHEM101: Diagnose The Acidity/ Alkalinity of Chemical Substances - “Acid Test” Chemistry Assessment Answers.
The assignments have been written by the professors who have PhD degree in the field of chemistry. Also, they have pursued their degrees from an Australian college which is beneficial for them in different ways. However, here is a sample for CHEM101: Diagnose The Acidity/ Alkalinity of Chemical Substances - “Acid Test” Chemistry Assessment and approaches to solve it.
TASK:

To solve the two parts of question 1, you are required to choose a different household product, the volume of each product, the volume of indicator, observed color and estimated pH.
 Let’s take an example of Red Cabbage Indicator
Experiment:
Let’s take an example of Red Cabbage Indicator
Experiment:
Step1: You have to first extract the purple dye. You will be required to tear the red cabbage leaves into small pieces or simply chop up the cabbage leaves or grind them.
Step2: After this, you need to put the tiny cabbage leaves into a jug. Then pour some hot water and leave it for few minutes. Now, you will see that the water gets turned into a bright purplish-blue colour.
Step3: Now, required to pipette a small amount of indicator solution. Also, add a different test compound to every beaker. You can use testing vinegar or sodium bicarbonate. Now, you will notice that the acid start to turn the alkali blue and indicator pink.
Step4: Now, put a few drops of each substance into that small indicator beakers and let it stirred. Wait for the change in colour. You will now come to find that the acids have been turned the indicator in the color like red or pink, and alkalis have turned it green, blue, or yellow.
Below is an example of red cabbage indicator examined on the basis of the above experiment:

After this, you can also prepare a diagram and fill with the observation. The table may look like:
| Step |
Substance Added |
Observation (e.g. colour) |
| 1. acid solution |
Lemon Juice |
|
| 2. Neutralization |
Sodium Bicarbonate |
|
| 3. Re-acidification |
Lemon Juice |
|
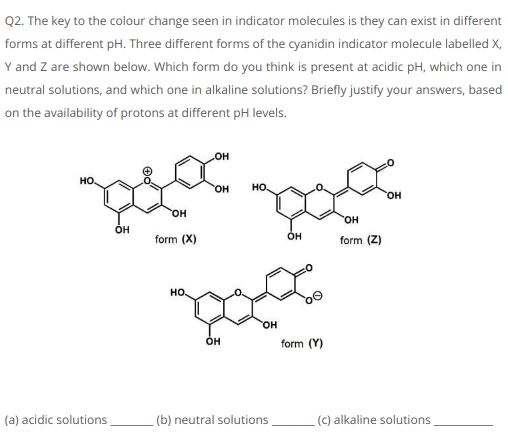 Approach To Solve CHEM101 Assignment (Question 2)
Approach To Solve CHEM101 Assignment (Question 2)
To answer the above question, you will be required to follow the following approach:
From the above diagram, it can be known that there are three forms i.e
- Acidic solutions which are in X form (pH <3)
- Neutral solutions in the form of Z (pH = 7-8)
- Alkaline solutions - Y form (pH >11)
Our CHEM101
assignment provider have concluded that at low pH, the accessibility of H+ particles in highly focused protonated the cyanidin molecule and it forms a positive ion (cation). Whereas with the increase in pH level, it expands the focus of H+ particles diminishes and also leads to deprotonation of cyanidin molecule and it frames a negative ion (anion).

In question 3 of your
CHEM101 assessment, you will be required to observe the experiment you have done earlier and find out whether the indicator molecule change from one form or another or not. If yes! Explain.
To provide an accurate answer for the above question, you must have the knowledge of principles that depends on the H+ particles. In case, you lack such principles answering further questions can be challenging.
Apart from this, there are few more questions like what will be the balanced chemical equation for the dissociation of CH3COOH in water, is acetic acid a strong acid or weak acid etc. Such type of questions is easy to answer. But there could be few issues while complete your CHEM101: ACID TEST CHEMISTRY ASSESSMENT. In such a situation, you can take help from SAMPLE ASSIGNMENT.
--
At
SAMPLE ASSIGNMENT, you will interact with the experts offering reliable
chemistry assignment help Australia at the lowest price. Additionally, you can also buy a sample for CHEM101: ACID TEST ASSESSMENT.
Recommended:

 To solve the two parts of question 1, you are required to choose a different household product, the volume of each product, the volume of indicator, observed color and estimated pH.
To solve the two parts of question 1, you are required to choose a different household product, the volume of each product, the volume of indicator, observed color and estimated pH.
 Let’s take an example of Red Cabbage Indicator
Experiment:
Step1: You have to first extract the purple dye. You will be required to tear the red cabbage leaves into small pieces or simply chop up the cabbage leaves or grind them.
Step2: After this, you need to put the tiny cabbage leaves into a jug. Then pour some hot water and leave it for few minutes. Now, you will see that the water gets turned into a bright purplish-blue colour.
Step3: Now, required to pipette a small amount of indicator solution. Also, add a different test compound to every beaker. You can use testing vinegar or sodium bicarbonate. Now, you will notice that the acid start to turn the alkali blue and indicator pink.
Step4: Now, put a few drops of each substance into that small indicator beakers and let it stirred. Wait for the change in colour. You will now come to find that the acids have been turned the indicator in the color like red or pink, and alkalis have turned it green, blue, or yellow.
Below is an example of red cabbage indicator examined on the basis of the above experiment:
Let’s take an example of Red Cabbage Indicator
Experiment:
Step1: You have to first extract the purple dye. You will be required to tear the red cabbage leaves into small pieces or simply chop up the cabbage leaves or grind them.
Step2: After this, you need to put the tiny cabbage leaves into a jug. Then pour some hot water and leave it for few minutes. Now, you will see that the water gets turned into a bright purplish-blue colour.
Step3: Now, required to pipette a small amount of indicator solution. Also, add a different test compound to every beaker. You can use testing vinegar or sodium bicarbonate. Now, you will notice that the acid start to turn the alkali blue and indicator pink.
Step4: Now, put a few drops of each substance into that small indicator beakers and let it stirred. Wait for the change in colour. You will now come to find that the acids have been turned the indicator in the color like red or pink, and alkalis have turned it green, blue, or yellow.
Below is an example of red cabbage indicator examined on the basis of the above experiment:
 After this, you can also prepare a diagram and fill with the observation. The table may look like:
After this, you can also prepare a diagram and fill with the observation. The table may look like:
 Approach To Solve CHEM101 Assignment (Question 2)
To answer the above question, you will be required to follow the following approach:
From the above diagram, it can be known that there are three forms i.e
Approach To Solve CHEM101 Assignment (Question 2)
To answer the above question, you will be required to follow the following approach:
From the above diagram, it can be known that there are three forms i.e
 In question 3 of your CHEM101 assessment, you will be required to observe the experiment you have done earlier and find out whether the indicator molecule change from one form or another or not. If yes! Explain.
To provide an accurate answer for the above question, you must have the knowledge of principles that depends on the H+ particles. In case, you lack such principles answering further questions can be challenging.
Apart from this, there are few more questions like what will be the balanced chemical equation for the dissociation of CH3COOH in water, is acetic acid a strong acid or weak acid etc. Such type of questions is easy to answer. But there could be few issues while complete your CHEM101: ACID TEST CHEMISTRY ASSESSMENT. In such a situation, you can take help from SAMPLE ASSIGNMENT.
--
At SAMPLE ASSIGNMENT, you will interact with the experts offering reliable chemistry assignment help Australia at the lowest price. Additionally, you can also buy a sample for CHEM101: ACID TEST ASSESSMENT.
Recommended:
In question 3 of your CHEM101 assessment, you will be required to observe the experiment you have done earlier and find out whether the indicator molecule change from one form or another or not. If yes! Explain.
To provide an accurate answer for the above question, you must have the knowledge of principles that depends on the H+ particles. In case, you lack such principles answering further questions can be challenging.
Apart from this, there are few more questions like what will be the balanced chemical equation for the dissociation of CH3COOH in water, is acetic acid a strong acid or weak acid etc. Such type of questions is easy to answer. But there could be few issues while complete your CHEM101: ACID TEST CHEMISTRY ASSESSMENT. In such a situation, you can take help from SAMPLE ASSIGNMENT.
--
At SAMPLE ASSIGNMENT, you will interact with the experts offering reliable chemistry assignment help Australia at the lowest price. Additionally, you can also buy a sample for CHEM101: ACID TEST ASSESSMENT.
Recommended:






Loved reading this Blog? Share your valuable thoughts in the comment section.
Add comment